PROVIDENCE, R.I. [Brown University] — Policymakers struggling to stop the spread of HIV grapple with “what if” questions on the scale of millions of people and decades of time. They need a way to predict the impact of many potential interventions, alone or in combination. In two papers to be presented at the 2012 International AIDS Society Conference in Washington, D.C., Brandon Marshall, assistant professor of epidemiology at Brown University, will unveil a computer program calibrated to model accurately the spread of HIV in New York City over a decade and to make specific predictions about the future of the epidemic under various intervention scenarios.
“It reflects what’s seen in the real world,” said Marshall. “What we’re trying to do is identify the ideal combination of interventions to reduce HIV most dramatically in injection drug users.”
In an analysis that he’ll present at 11 a.m. in Session Room 6 on Friday, July 27, Marshall projects that with no change in New York City’s current programs, the infection rate among injection drug users will be 2.1 per 1,000 in 2040. Expanding HIV testing would drop the rate only 12 percent to 1.9 per 1,000; increasing drug treatment would reduce the rate 26 percent to 1.6 per 1,000; providing earlier delivery of antiretroviral therapy and better adherence would drop the rate 45 percent to 1.2 per 1,000; and expanding needle exchange programs would reduce the rate 34 percent to 1.4 per 1,000. Most importantly, doing all four of those things would cut the rate by more than 60 percent, to 0.8 per 1,000.

“These results show that a comprehensive set of proven interventions must be scaled up immediately if we are to substantially reduce the spread of HIV among drug users,” Marshall said.
Virtual reality, real choices
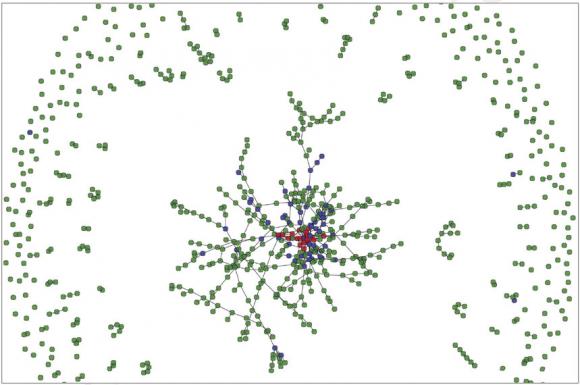
The model is unique in that it creates a virtual reality of 150,000 “agents,” a programming term for simulated individuals, who in the case of the model, engage in drug use and sexual activity like real people.
Like characters in an all-too-serious video game, the agents behave in a world governed by biological rules, such as how often the virus can be transmitted through encounters such as unprotected gay sex or needle sharing.
With each run of the model, agents accumulate a detailed life history. For example, in one run, agent 89,425, who is male and has sex with men, could end up injecting drugs. He participates in needle exchanges, but according to the built-in probabilities, in year three he shares needles multiple times with another injection drug user with whom he is also having unprotected sex. In the last of those encounters, agent 89,425 becomes infected with HIV. In year four he starts participating in drug treatment and in year five he gets tested for HIV, starts antiretroviral treatment, and reduces the frequency with which he has unprotected sex. Because he always takes his HIV medications, he never transmits the virus further.
That level of individual detail allows for a detailed examination of transmission networks and how interventions affect them.
“With this model you can really look at the microconnections between people,” said Marshall, who began working on the model as a postdoctoral fellow at Columbia University and has continued to develop it since coming to Brown in January. “That’s something that we’re really excited about.”
To calibrate the model, Marshall and his colleagues found the best New York City data they could about how many people use drugs, what percentage of people were gay or lesbian, the probabilities of engaging in unprotected sex and needle sharing, viral transmission, access to treatment, treatment effectiveness, participation in drug treatment, progression from HIV infection to AIDS, and many more behavioral, social and medical factors. They also continuously calibrated it until the model could faithfully reproduce the infection rates among injection drug users that were known to occur in New York between 1992 and 2002.
And they don’t just run the simulation once. They run it thousands of times on a supercomputer at Brown to be sure the results they see are reliable.
Future applications
At Brown, Marshall is continuing to work on other aspects of the model, including an analysis of the cost effectiveness of each intervention and their combinations. Cost is, after all, another fact of life that policymakers and public health officials must weigh.
And then there’s the frustrating insight that the infection rate, even with four strengthened interventions underway, didn’t reduce the projected epidemic by much more than half.
“I actually expected something larger,” Marshall said. “That speaks to how hard we have to work to make sure that drug users can access and benefit from proven interventions to reduce the spread of HIV.”
Marshall’s collaborators on the model include Magdalena Paczkowski, Lars Seemann, Barbara Tempalski, Enrique Pouget, Sandro Galea, and Samuel Friedman.
The National Institutes of Health and the Lifespan/Tufts/Brown Center for AIDS Research provide financial support for the model’s continued development.