PROVIDENCE, R.I. [Brown University] — Harmful bacteria have evolved some ingenious mechanisms to resist antibiotics. One of those is the drug efflux pump — proteins that stand guard along bacterial cell membranes, identifying antibacterial agents that pass through the membrane and swiftly ejecting them from the cell.
“These drug efflux pumps are extremely problematic,” said Jason Sello, associate professor of chemistry at Brown University. “The drugs are pumped out of the bacteria and cannot reach the critical concentration for toxicity.”
Sello and a team of researchers from Brown have come up with a new strategy that may help sneak drugs past the efflux guards. The new approach makes use of molecular fragments administered alongside antimicrobial agents. The efflux pumps are kept busy pumping out the fragments while the antimicrobial agents are able to stay inside the cell.
“We’re basically using decoys,” Sello said. “It’s a relatively simple idea to solve a significant problem in medicine.”
Sello and his colleagues describe the method and some preliminary lab results in a paper published in the journal ACS Infectious Diseases. The paper was co-authored by graduate students Corey Compton and Daniel Carney and undergraduate Patrice Groomes.
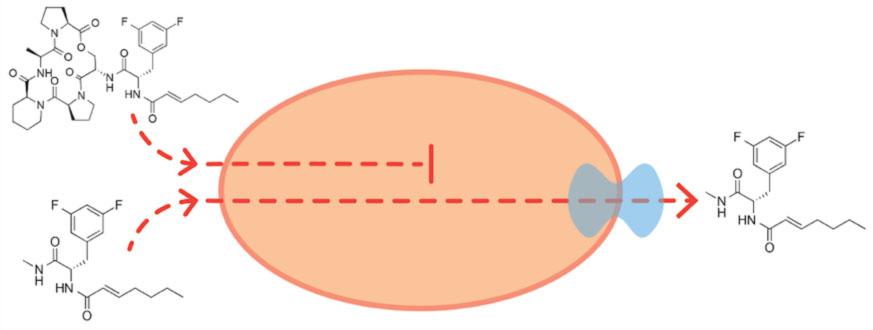
For the study, Sello and his team experimented with a promising new class of antimicrobial drug candidates called acyldepsipeptides or ADEPs. The compounds have been shown to be effective in killing many species of bacterial pathogens but are generally less effective against the bacterium that causes tuberculosis. It had been reported that the ADEP resistance of M. tuberculosis was due to the presence of one or perhaps more efflux pumps. Sello and his team were seeking a way to interfere with those pumps so that the ADEPs could be used for the treatment of tuberculosis.
“There are two scenarios for how an ADEP efflux pump could operate,” Sello said. “The pump could either recognize the entire molecule or some portion of it. We thought, if the latter scenario is operative, then a molecule comprising the minimal portion of the ADEP that is recognized by the pump could competitively interfere with efflux of the ADEP.”
Sello and his team went to work synthesizing molecules that were fragments of the larger ADEP molecule. They then placed the fragments, along with the full ADEPs, in test tubes containing Streptomyces coelicolor, a nonpathogenic relative of M. tuberculosis that is safe to work with in the laboratory. They found that one of the fragments — a molecule matching the side chain of the full ADEP molecule — increased the efficacy of the ADEP four-fold in killing the bacteria.
To make sure that the capacity of the fragment to synergize the ADEP was a result of competitive interference with efflux, the researchers repeated the experiment in a genetically engineered strain of S. coelicolor in which genes encoding an efflux pump were over-expressed. This strain was highly resistant to the ADEP alone. However, the ADEP susceptibility of the strain significantly increased when the molecular decoy was used in the experiment. That’s a pretty clear sign that the decoys are indeed interfering with the efflux pump.
Follow-up experiments carried out with M. tuberculosis showed similar results. In fact, the team showed that the fragment approach worked better than combining ADEPs with reserpine, a well-known efflux inhibitor.
“One of the things that surprised us in this case was the fact that you didn’t need much of the fragment to enhance the potency of the ADEP,” Sello said. “This result suggests that fragment is preferentially acted upon by the pump.”
Sello says these initial experiments provide “a strong proof of principle” for a new approach to suppressing drug efflux. Ultimately, the researchers hope it might be possible to design a drug that delivers both antibacterial agents and decoy molecules.
The strategy could also be applied in the treatment of cancer, Sello said. Some cancers do not respond to drugs because cancerous cells have efflux pumps. The team is optimistic that this strategy could find broad use in chemotherapy.
“Efflux pumps are part of a nightmare scenario in the treatment of infectious diseases,” Sello said. “There are efflux pumps that can export multiple drugs that have different structures and mechanisms of action. If a pathogenic strain of bacteria acquires a gene encoding one of these efflux pumps, then it will suddenly become resistant to multiple antibacterial drugs. Since an efflux pump could compromise the potency of even a newly developed antibacterial drug, we need to think about ways to circumvent these efflux pumps.”
This latest work suggests a promising strategy for doing just that.