PROVIDENCE, R.I. [Brown University] — Because Brown University biomedical engineering graduate student Molly Boutin needed to study how neural tissues grow from stem cells, she wanted to grow not just a cell culture, but a sphere-shaped one. Cells grow and interact more naturally in 3-D cultures than when they’re confined to thin slides or dishes.
But the very advantage of a culture having thickness also poses a challenge: How to see all the cells and their connections all the way through the culture. It’s a problem that confronts many biologists, physicians, bioengineers, drug developers- and others who also see 3-D cultures as a useful stage before moving to animal models.
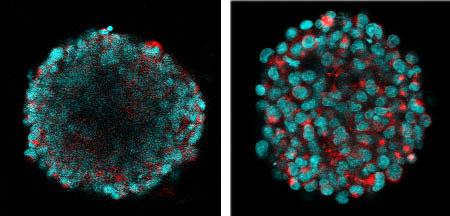
“As I was imaging these tissues I was only able to get the outer layer or two of cells and that wasn’t a very good representation of what was going on inside of the sphere,” Boutin said.
There are inelegant ways to slice up an engineered 3-D tissue for imaging and then to reconstruct it, but a more tantalizing solution seemed likely to come from one of the chemical treatments invented in just the last few years to make tissues see-through. But which one, if any, would work with her scaffold-free engineered neural tissues? To find out, Boutin and adviser, Diane Hoffman-Kim, associate professor of medical science in the Department of Molecular Pharmacology, Physiology, and Biotechnology, decided to test the three simplest methods: ClearT2, SeeDB and Scale.
Their results – which read like a Consumer Reports article for the lab bench set – now appear online in the journal Tissue Engineering Part C: Methods.
Using a confocal microscope, researchers can study a cleared spherical 3-D tissue culture at any depth. The video begins on the near surface and exits on the far side of the culture.
For Boutin’s critieria, the ClearT2 method turned out to be clear winner. For her little balls of neural tissue – 100 millionths of a meter in diameter – ClearT2 allowed her to see fluorescing cells at all depths of focus and, importantly, it did not change the size of the tissue. Scale made her “neural spheres” substantially larger, while SeeDB made them smaller and didn’t improve clarity as much.
Maintaining the tissue culture size is important because Boutin wants to know the physical dimensions of growth in the culture, such as the axons that one neuron might extend to another.
While ClearT2 worked within 1.5 hours, Scale and SeeDB took three days, Boutin said.
She ran several further tests with ClearT2, including with other types of healthy and cancerous neural cells, and ClearT2 continued to perform well, even allowing her to image extracellular matrix.
In the paper Boutin and Hoffman-Kim acknowledge that the results with different methods may vary with different samples, but they expect that for many “scaffold-free” engineered 3-D neural tissues – tissues that grow without added matrix supports – ClearT2 should work well.
Knowing that could clear the way for many research projects with 3-D tissues.
The National Science Foundation, The National Institutes of Health, and the Brown Institute for Brain Science supported the research. Imaging occurred in Brown’s Leduc Bioimaging Facility. Boutin created her 3-D cultures with technology from Microtissues Inc., a company founded by Jeffrey Morgan, professor of medical science in the Department of Molecular Pharmacology, Physiology, and Biotechnology at Brown.