PROVIDENCE, R.I. [Brown University] — Nicotine isn’t just addictive. It may also interfere with dozens of cellular interactions in the body, new Brown University research suggests.
Conversely, the data could also help scientists develop better treatments for various diseases. Pharmaceutical companies rely on basic research to identify new cellular interactions that can, in turn, serve as targets for potential new drugs.
“It opens several new lines of investigation,” said lead author Edward Hawrot, professor of molecular science, molecular pharmacology, physiology and biotechnology at Brown University.
Hawrot’s research is highlighted in a paper published April 3 in the Journal of Proteome Research. He and a team that included graduate students William Brucker and Joao Paulo set out to provide a more basic understanding of how nicotine affects the process of cell communication through the mammalian nervous system.
The Brown University researchers looked specifically at the alpha-7 nicotinic acetylcholine receptor in mouse brain tissue. A very similar receptor exists in humans. The alpha-7 receptor is the most enigmatic of the so-called “nicotinic” receptors, so named because nicotine binds to them when it is introduced into the body. Most receptors are on the surface of cells and are sensitive to small signaling molecules such as the neurotransmitter acetylcholine, which is the naturally occurring signal the body uses to activate alpha-7 receptors.
Their discovery: 55 proteins were found to interact with the alpha-7 nicotinic receptor. Scientists had not previously known of those connections.
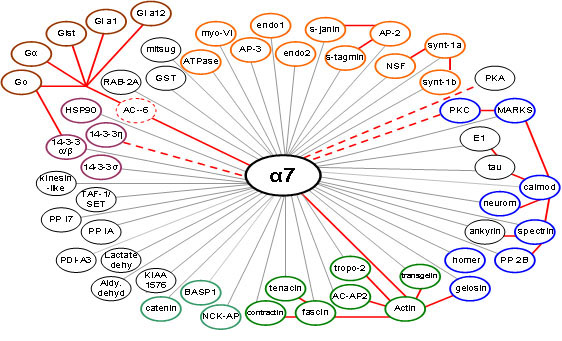
“This is called a “nicotinic” receptor and we think of it as interacting with nicotine, but it likely has multiple functions in the brain,” Hawrot said. “And in various, specific regions of the brain this same alpha-7 receptor may interact with different proteins inside neurons to do different things.”
One in particular — the G alpha protein — was among the most unexpected proteins to be identified in the study, as it is usually associated with a completely different class of receptors (the eponymous G-protein coupled receptors (GPCRs).
This finding is significant because G alpha proteins are involved in many different biochemical and signaling processes throughout the brain and the rest of the body. body.
An example of the importance of G alpha proteins: 40 percent of all currently used therapeutic drugs target a member of the large GPCR family of receptors.
The new finding suggests that the alpha-7 receptors have a much broader role in the body than previously suspected and that the newly identified associated proteins could also be affected when nicotine binds to the alpha-7 receptor.
Nicotine may affect bodily processes — and perhaps the actions of other commonly used drugs — more broadly than was previously thought.
This advance could lead to the development of new treatments to combat smoking addiction. At the same time, the finding could also have future implications for diseases such as schizophrenia, Hawrot said.
Recent genetic studies have suggested that some cases of schizophrenia are associated with deletions where a block of genes, including the gene for the alpha-7 receptor, is missing. Hawrot said the connection, while not conclusive, offers hope for new strategies in the development of treatments for those suffering from the disorder.
To conduct their study, Hawrot’s lab looked at mice genetically engineered by other researchers to lack the alpha-7 nicotinic acetylcholine receptor. Those mice were compared with normal mice, so the difference in receptor-associated proteins could be highlighted.
Grants from the National Institutes of Health and the Rhode Island Research Alliance helped support the study.

