PROVIDENCE, R.I. [Brown University] — Gray mold is a gardener’s nightmare. The fungus, also known by its scientific name Botrytis cinerea, is a scourge to more than 200 agricultural and ornamental plant species, including staples such as tomatoes, strawberries, snap and lima beans, cabbage, lettuce and endive, peas, peppers, and potatoes. Gray mold envelops its target in a velvety vise, releasing a toxin that poisons the host plants’ cells, eventually causing the plant to die.
So far, the only way to eliminate the pathogen is to spray plants with fungicides, which can be costly and can contaminate the surrounding environment.
Now Brown University chemist David Cane, working with researchers in France and Spain, has figured out how the fungus’s deadly toxin is made and how it might be disarmed naturally. In a paper published online in ACS Chemical Biology, the scientists have identified the set of genes that manufactures the toxin and in particular the central gene the fungus uses for this synthesis. They also have also shown that shutting off this gene by interrupting the fungus’s DNA completely shuts down toxin production, removing the special weapon the mold uses to kill and invade target plant cells.
“It’s a big step to being able to disarm this toxin naturally through a combination of DNA sequencing and chemistry,” said Cane, the Vernon K. Krieble Professor of Chemistry and professor of biochemistry, one of three primary authors of the paper.
The researchers, led by French scientist and paper co-author Muriel Viaud, started by determining the complete DNA sequence for Botrytis cinerea. Working with Spanish organic chemist and paper co-author Isidro Collado, the scientists focused on the chemical agent — botrydial — that gray mold uses to overwhelm host plants.
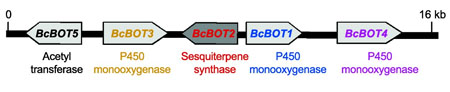
From among the roughly 9,000 genes present in gray mold, the researchers identified a cluster of five genes that is responsible for production of botrydial. They then sought to learn how this cluster manufactures the chemical agent and which of the genes was the mastermind in the production.
The culprit is an enzyme called a sesquiterpene cyclase, Cane’s laboratory found.
“The metabolic pathways for creating organic compounds typically involve gene clusters, like a package,” Cane explained. “One great advantage to our investigation is that if you find one, you look to the left or to the right, and you find the others.”
In laboratory tests, Cane and the team introduced a mutant gene that deleted the sesquiterpene cyclase, which completely abolished production of the toxin.
“This means that if you can inhibit the enzyme from this pathway, you can eliminate this toxin,” Cane said.
The U.S. National Institutes of Health, the INRA Jeune Equipe in France and the Ministry of Education and Science in Spain funded the research.
The team now is working on a similar procedure to tackle a strain of Botrytis cinerea that is able to produce both botrydial and a second toxin that it uses to attack its plant targets.

