PROVIDENCE, R.I. [Brown University] — With rising energy prices and greater concern over global warming, compact fluorescent lamps (CFLs) are having a successful run. Sales of the curlicue, energy-sipping bulbs, which previously had languished since they were introduced in the United States in 1979, reached nearly 300 million last year. Experts expect that figure to rise steeply by 2012, when a federal law requiring energy-efficient lighting goes into effect.
There’s just one catch to this energy conservation story: Each CFL contains a small amount (3 to 5 milligrams) of mercury, a neurotoxin that can be released as vapor when a bulb is broken. The gas can pose a minor risk to certain groups, such as infants, small children and pregnant women. Mercury can escape from plastic bags containing discarded bulbs, which makes long-term storage, disposal or recycling tricky.
The obstacles have led to a debate over CFLs, illustrated by recent studies by the state of Maine and the nonprofit Mercury Policy Project over CFL use and safe levels of mercury in the bulbs. Now, a team of researchers at Brown University led by Robert Hurt, professor of engineering, and engineering student Natalie Johnson may have found a solution to the environmental conundrum.
The scientists, along with other Brown engineering students and Steven Hamburg, associate professor of environmental studies, have invented mercury-absorbent materials for commercial use. The team has created a prototype – a mercury-capturing lining attached to the inside of store-bought CFL packaging. The packaging can be placed over the area where a bulb has been broken to absorb the mercury vapor emanating from the spill, or it can capture the mercury of a bulb broken in the box.
The researchers also have created a specially designed lining for plastic bags that soaks up the mercury left over from the CFL shards that are thrown away.
The mercury-absorbent packaging and the lined plastic bags can be safely discarded and recycled, the researchers say, alleviating concerns about contamination or other unwanted environmental consequences.
“It’s a complete management system to deal with a bulb broken in the home,” says Hurt, director of Brown’s Institute for Molecular and Nanoscale Innovation, which concentrates on the study and commercial application of nanotechnology.
Brown applied earlier this year for federal patents covering the mercury-absorption packaging and the absorbent material, and the university expects soon to begin discussions with companies on manufacturing the new technology.
“These patents represent how Brown University translates fundamental research into an application that can have an impact on society – in this case, a technology that could protect households from mercury exposure and that could also energize green business growth,” says Clyde Briant, vice president for research at Brown.
The inspiration for the invention followed the discovery by Hurt, Johnson and fellow Brown researchers that a variant of a substance called nanoselenium – a form of selenium, a trace element used in diet supplements, among other products – absorbed virtually all the mercury emitted from a broken CFL. That finding appears this week in the online edition of Environmental Science & Technology. It is the first scientific paper that measures the timing and extent of mercury released from broken CFLs and that reveals the mercury-absorption potential of various nanomaterials, the researchers say.
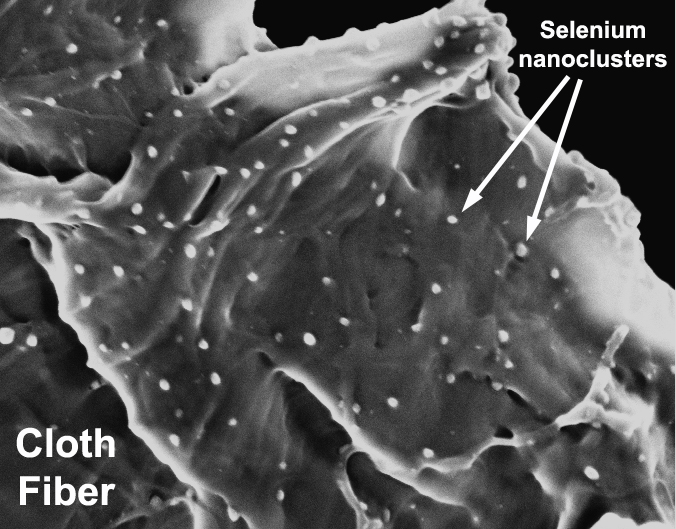
The engineers tested 28 substances in all. Their experiments showed that one type of nanoselenium absorbed mercury vapor the most effectively. The selenium atoms bond with the mercury atoms to form mercury selenide (HgSe), a stable, benign nanoparticle compound, Hurt says.
The nanoselenium “just loves mercury,” Hurt adds.
In controlled experiments, the scientists found that 99 percent of mercury vapor from a CFL broken in a sealed chamber was mopped up by nanoselenium in concentrations ranging from 1 to 5 milligrams.
The small amount needed to capture the mercury vapor bodes well for manufacturing mercury-absorbent cloths or lining at a low cost, Hurt says. The precise manufacturing costs will need to be determined by interested companies.
The National Institute of Environmental Health Sciences Superfund Basic Research Program funded the research.
The first prototype created by the Brown team is a three-layered cloth that is attached to the packaging or box containing the CFLs. The nanoselenium-coated layer would be sandwiched between the cardboard packaging and a cloth on the inside of the box containing the bulbs. The extra layers prevent people from coming into contact with the nanoselenium layer.
If a bulb breaks, the user simply undoes the packaging and lays it on the spot where the break occurred. The absorbent material is effective on different surfaces, including carpets and hardwood floors. “It works like a charm,” Hurt says.
The second prototype incorporates the same layering and is fitted into a small, sealable plastic bag. The lining absorbs the mercury in the sealed bag, preventing it from escaping.
“More work is needed,” Hurt says, “but this appears to be an inexpensive solution that can remove most of the safety concerns associated with CFL bulbs.”