PROVIDENCE, R.I. [Brown University] — Biologists at Brown University have found a way to measure the effects of aging by watching the ebb and flow of chromatin, a structure along strands of DNA that either silences or permits gene expression. In several newly published experiments they show that gene silencing via chromatin in fruit flies declines with age.
They also showed that administering life span extending measures to the flies, such as switching them to a lower calorie diet or increasing expression of the protein Sir2, restores the observed loss of gene silencing due to age.
“For many years it has been suggested that one of the issues that occurs with age, leading to cellular dysfunction, is that some genes that should be silenced lose that silencing,” said Dr. Stephen Helfand, senior author of the study published online Nov. 15 in the journal Aging. “It hasn’t been very well demonstrated to take place other than in yeast. So what we were trying to do in flies is see whether genes that are normally repressed lose their repression.”
The answer they report is that the phenomenon is true in flies too.
The variegations of age
To achieve those findings, Helfand and lead author Nan Jiang exploited a phenomenon called “position effect variegation.” PEV is the variation of a gene’s expression that comes from the gene existing at the border between euchromatin, a loose wrapping of DNA that readily permits gene expression, and heterochromatin, a tight wrapping that keeps it locked down. The scientists hypothesized that as organisms age, heterochromatin might recede, allowing more genes that had once been silenced to become exposed for expression — like a seashell becoming revealed to a passing beachcomber as the tide recedes.

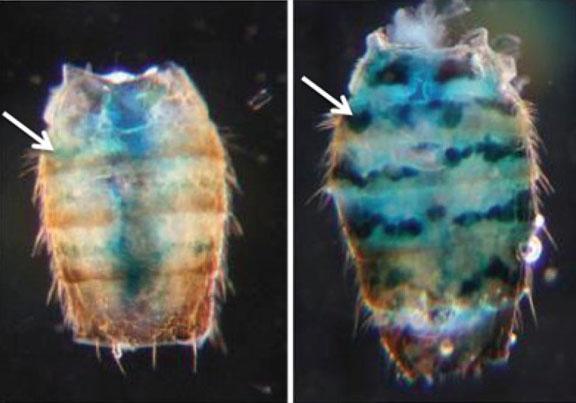
To test whether chromatin gene silencing declines with age, the researchers inserted “reporter” genes right at the border between heterochromatin and euchromatin in two specific parts of the flies’ genomes. The reporter genes have the useful property of showing blue when expressed. If they aren’t being silenced, their expression can be verified simply by applying a brief pulse of heat to the fly. The technique gave the researchers an indicator of chromatin-related gene expression in individual cells, in individual tissues at any time during the life cycle of the fly.
As flies aged, the team found they became increasingly likely to express the reporter gene in those border regions between heterochromatin and euchromatin. For example, at 10 days of age 5 to 35 percent of cells in certain tissues showed expression of the reporter gene, but at 50 days of age, 40 to 80 percent of cells in those tissues showed expression.
The team, including Guyu Du and Ethan Tobias, who were Brown undergraduates at the time, also directly measured the extent of heterochromatin in the regions of the inserted genes over time. They found that heterochromatin in those areas indeed lessened with age.
Altering aging?
The next set of experiments showed that while time is relentless, this particular manifestation of aging was more flexible. The team treated some of their flies with a couple of interventions that in previous research has been shown to extend life span. They wanted to see whether they affected heterochromatin and gene silencing.
One intervention was to overexpress the gene that produces an enzyme called Sir2. In flies where they did that, gene silencing increased. The other intervention was to put flies on a reduced calorie diet. That, too, forestalled the decline of gene silencing with age.
In one last experiment, the researchers gave some flies a high-calorie diet and some a low-calorie diet and then switched their diet either at 20 or 30 days of age. What they found was that within 72 hours of switching diets, the flies’ levels of gene silencing came to resemble that of flies who had been on that diet all along. In other words, a fly newly brought to a low-calorie diet would have the relatively high silencing of a fly that had always been on a low-calorie diet.
That result suggests that while gene silencing appears to erode with age naturally, it can be throttled back and forth with certain interventions.
“This gets at one molecular mechanism by which aging might be put off,” said Helfand, who teaches in Brown’s Department of Molecular Biology, Cell Biology and Biochemistry.
In a recent study led by colleague Robert Reenan, Helfand showed that reducing RNA editing and thereby preserving heterochromatin in flies increased life span.
The fundamental biology he’s observed in flies has a good chance of also being at play in humans, Helfand said. He noted a paper earlier this year by Brown colleague John Sedivy, who along with mutual co-author Nicola Neretti, saw evidence in humans for a breakdown in heterochromatin and genetic control with age.
Still, humans would not tolerate interventions that work for flies, such as severe calorie restriction. Instead, the research suggests a possible line of research to develop more practical interventions.
Finding benign ways to mimic the effects of calorie restriction to restore overall gene silencing, for instance, could ultimately become an alternative to treating the effects of unwanted expression of individual genes in aging patients.
“We’re trying to return the cell to a more youthful heterochromatin state with the hope that that will improve normal cell physiology and combat the loss of cellular function with age,” Helfand said.
Additional authors on the paper are Jason Wood and Rachel Whitaker.
The National Institute on Aging and the Ellison Medical Foundation funded the research.