PROVIDENCE, R.I. [Brown University] — In a new study, scientists report that they substantially curbed weight gain, improved metabolism, and improved the efficacy of insulin in mice by engineering them to express a specific human enzyme in their fat tissue. Although the obesity prevention came at the significant cost of widespread inflammation, the research offers new clues about the connections among obesity, insulin resistance and type 2 diabetes, and inflammation.
“Turning on this molecule has a very dramatic impact on lipid metabolism,” said Haiyan Xu, assistant professor of medicine (research) in the Warren Alpert Medical School of Brown University and a researcher at Rhode Island Hospital’s Hallett Center for Diabetes and Endocrinology. Xu is the corresponding author of a paper describing the research in the January 2012 issue of Endocrinology and released early online.

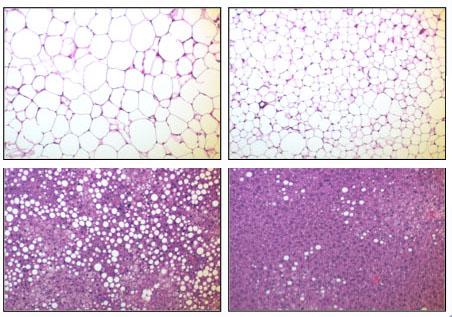
The relationship between fat, inflammation, and insulin performance is complex. The conventional wisdom is that obesity leads to inflammation which contributes to insulin resistance. In this study, the researchers changed the sequence of events for transgenically engineered mice by inducing inflammation via the enzyme IKKbeta in their fatty tissue before they were obese. The result for metabolism was much more positive than for control mice who were left unaltered but were fed the same diets.
For both male and female mice, the ones who were altered still put on weight but significantly more slowly. All the mice started at the same weight. After about 22 weeks on a high-fat diet, however, altered male mice weighed less than 38 grams while unaltered male mice weighed more than 45 grams. On a less extravagant diet named “chow” the difference was considerably lessened but was still statistically significant. Both trends held for females as well.
The altered mice experienced slower weight gain despite eating much more food. Their increased metabolism allowed them to dispatch the extra calories much more efficiently. After being injected with glucose, for example, altered mice maintained lower blood sugar levels than unaltered mice. The same was true after insulin injections, suggesting that insulin was more effective. In addition, the transgenic mice expended much more energy than their normal counterparts, suggesting that the sugar was indeed metabolized.
The mechanisms by which IKKbeta in fatty tissue increases metabolic performance are not completely clear, but the researchers measured increased expression of genes associated both with fatty acid oxidation and with making mitochondria, a cell part responsible for producing energy.
One possible lesson from the research seems to be that while obesity and inflammation are both promoters of insulin resistance, Xu said, obesity seems to be the worse one.
“Lower body weight is always a beneficial thing for influencing insulin sensitivity,” she said. “Reduced adiposity wins over increased inflammation.”
Another point is that IKKbeta’s ability to aid metabolism may be specific to its activation in fat tissue. In previous studies, scientists had activated it in the liver with no weight-reduction benefits and in the brain’s hypothalamus, leading to increased weight gain.
The paper’s lead author is research fellow Ping Jiao also of the Hallett Center and Brown. Other authors were Bin Feng, Yaohui Nie, and Yujie Li of the Hallett Center; Jie Ma of Rhode Island Hospital and the Department of Medicine in the Alpert Medical School; and Erin Paul of Brown’s Department of Molecular Biology, Cell Biology, and Biochemistry. Jiao and Ma are also affiliated with the Jilin University in China and Bin Feng is also affiliated with Huazhong Agricultural University in China.
Funding for the research came from the American Heart Association, which provided Xu with a scientist development grant, and Brown University, which awarded Jiao the George Bray fellowship.