PROVIDENCE, R.I. [Brown University] — Demonstrating an important milestone for the longevity and utility of implanted brain-computer interfaces, a woman with tetraplegia using the investigational BrainGate* system continued to control a computer cursor accurately through neural activity alone more than 1,000 days after receiving the BrainGate implant, according to a team of physicians, scientists, and engineers developing and testing the technology at Brown University, the Providence VA Medical Center, and Massachusetts General Hospital (MGH). Results from five consecutive days of device use surrounding her 1,000th day in the device trial appeared online March 24 in the Journal of Neural Engineering.
“This proof of concept — that after 1,000 days a woman who has no functional use of her limbs and is unable to speak can reliably control a cursor on a computer screen using only the intended movement of her hand — is an important step for the field,” said Dr. Leigh Hochberg, a Brown engineering associate professor, VA rehabilitation researcher, visiting associate professor of neurology at Harvard Medical School, and director of the BrainGate pilot clinical trial at MGH.
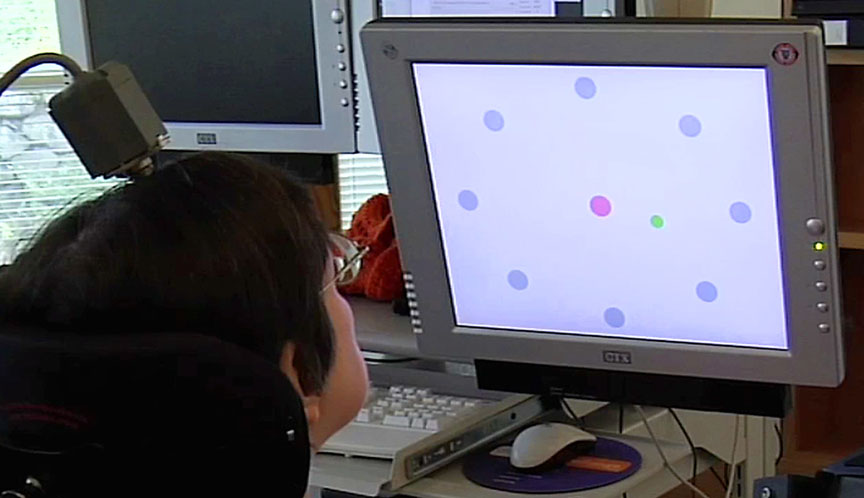
The woman, identified in the paper as S3, performed two “point-and-click” tasks each day by thinking about moving the cursor with her hand. In both tasks she averaged greater than 90 percent accuracy. Some on-screen targets were as small as the effective area of a Microsoft Word menu icon.
“Our objective with the neural interface is to reach the level of performance of a person without a disability using a mouse,” said report lead author John Simeral, a VA researcher and assistant professor of engineering at Brown. “These results highlight the potential for an intracortical neural interface system to provide a person that has locked-in syndrome with reliable, continuous point-and-click control of a standard computer application.”
In each of S3’s two tasks, performed in 2008, she controlled the cursor movement and click selections continuously for 10 minutes. The first task was to move the cursor to targets arranged in a circle and in the center of the screen, clicking to select each one in turn. The second required her to follow and click on a target as it sequentially popped up with varying size at random points on the screen.
From fundamental neuroscience to clinical utility
Under development since 2002, the investigational BrainGate system is a combination of hardware and software that directly senses electrical signals produced by neurons in the brain that control movement. By decoding those signals and translating them into digital instructions, the system is being evaluated for its ability to give people with paralysis control of external devices such as computers, robotic assistive devices, or wheelchairs. The BrainGate team is also engaged in research toward control of advanced prosthetic limbs and toward direct intracortical control of functional electrical stimulation devices for people with spinal cord injury, in collaboration with researchers at the Cleveland FES Center.
The system is currently in pilot clinical trials, directed by Hochberg at MGH.
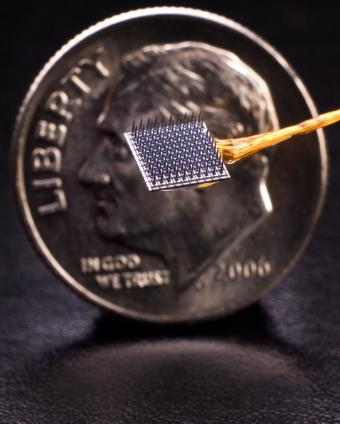
BrainGate uses a tiny (4x4 mm, about the size of a baby aspirin) silicon electrode array to read neural signals directly within brain tissue. Although external sensors placed on the brain or skull surface can also read neural activity, they are believed to be far less precise. In addition, many prototype brain implants have eventually failed because of moisture or other perils of the internal environment.
“Neuroengineers have often wondered whether useful signals could be recorded from inside the brain for an extended period of time,” Hochberg said. “This is the first demonstration that this microelectrode array technology can provide useful neuroprosthetic signals allowing a person with tetraplegia to control an external device for an extended period of time.”
Moving forward
Device performance was not the same at 2.7 years as it was earlier on, Hochberg added. At 33 months fewer electrodes were recording useful neural signals than after only six months. But John Donoghue — VA senior research career scientist, Henry Merritt Wriston Professor of Neuroscience, director of the Brown Institute for Brain Science, and original developer of the BrainGate system — said no evidence has emerged of any fundamental incompatibility between the sensor and the brain. Instead, it appears that decreased signal quality over time can largely be attributed to engineering, mechanical or procedural issues. Since S3’s sensor was built and implanted in 2005, the sensor’s manufacturer has reported continual quality improvements. The data from this study will be used to further understand and modify the procedures or device to further increase durability.
“None of us will be fully satisfied with an intracortical recording device until it provides decades of useful signals,” Hochberg said. “Nevertheless, I’m hopeful that the progress made in neural interface systems will someday be able to provide improved communication, mobility, and independence for people with locked-in syndrome or other forms of paralysis and eventually better control over prosthetic, robotic, or functional electrical stimulation systems [stimulating electrodes that have already returned limb function to people with cervical spinal cord injury], even while engineers continue to develop ever-better implantable sensors.”
In addition to demonstrating the very encouraging longevity of the BrainGate sensor, the paper also presents an advance in how the performance of a brain-computer interface can be measured, Simeral said. “As the field continues to evolve, we’ll eventually be able to compare and contrast technologies effectively.”
As for S3, who had a brainstem stroke in the mid-1990s and is now in her late 50s, she continues to participate in trials with the BrainGate system, which continues to record useful signals, Hochberg said. However, data beyond the 1000th day in 2008 has thus far only been presented at scientific meetings, and Hochberg can only comment on data that has already completed the scientific peer review process and appeared in publication.
In addition to Simeral, Hochberg, and Donoghue, other authors are Brown computer scientist Michael Black and former Brown computer scientist Sung-Phil Kim.
About the BrainGate collaboration
This advance is the result of the ongoing collaborative BrainGate research at Brown University, Massachusetts General Hospital, and Providence VA Medical Center. The BrainGate research team is focused on developing and testing neuroscientifically inspired technologies to improve the communication, mobility, and independence of people with neurologic disorders, injury, or limb loss.
For more information, visit www.braingate2.org.
The implanted microelectrode array and associated neural recording hardware used in the BrainGate research are manufactured by BlackRock Microsystems, LLC (Salt Lake City, UT).
This research was funded in part by the Rehabilitation Research and Development Service, Department of Veterans Affairs; The National Institutes of Health (NIH), including NICHD-NCMRR, NINDS/NICHD, NIDCD/ARRA, NIBIB, NINDS-Javits; the Doris Duke Charitable Foundation; MGH-Deane Institute for Integrated Research on Atrial Fibrillation and Stroke; and the Katie Samson Foundation.
The BrainGate pilot clinical trial was previously directed by Cyberkinetics Neurotechnology Systems, Inc., Foxborough, MA (CKI). CKI ceased operations in 2009. The clinical trials of the BrainGate2 Neural Interface System are now administered by Massachusetts General Hospital, Boston, Mass. Donoghue is a former chief scientific officer and a former director of CKI; he held stocks and received compensation. Hochberg received research support from Massachusetts General and Spaulding Rehabilitation Hospitals, which in turn received clinical trial support from Cyberkinetics. Simeral received compensation as a consultant to CKI.
* CAUTION: Investigational Device. Limited by Federal Law to Investigational Use.