PROVIDENCE, R.I. [Brown University] — Although the incidence of malaria has declined in all but a few countries worldwide, according to a World Health Organization report earlier this month, malaria remains a global threat. Nearly 800,000 people succumbed to the mosquito-borne disease in 2009, nearly all of them in the developing world.
Physicians do not have reliable treatment for the virus at various stages, largely because no one has been able to document the malaria parasite’s journeys in the body.
Now researchers at Brown University and the Massachusetts Institute of Technology have used advanced computer modeling and laboratory experiments to show how malaria parasites change red blood cells and how the infected cells impede blood flow to the brain and other critical organs.
Their findings, published in the early online edition of the Proceedings of the National Academy of Sciences, could help doctors chart, in real time, the buildup in the body of cells infected with malaria or other diseases (such as sickle-cell anemia) and to prescribe treatment accordingly.
“The idea is to predict the evolution of these diseases, just like we predict the weather,” said George Karniadakis, professor of applied mathematics at Brown and corresponding author on the paper.
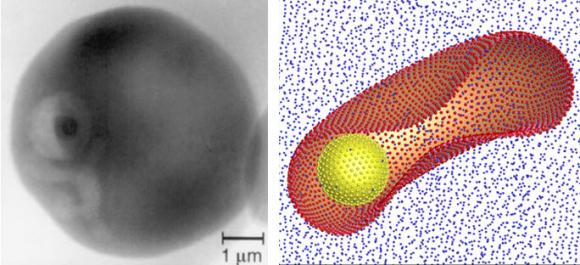
The researchers worked with Plasmodium falciparum, a parasite that can cause cerebral malaria by lodging in capillaries of the brain, especially among children. The parasite is found globally but is most common in Africa.
Once introduced into the human body by an infected mosquito’s bite, the parasite invades red blood cells. Healthy red blood cells are tremendously elastic; even though they can reach 8 microns in length and 2 microns in thickness, they can easily slide through a capillary just 3 microns in diameter. Capillaries are vital conduits in the human brain and other organs; red blood cells are key transporters of oxygen and nutrients.
Through extensive modeling carried out on one of the world’s fastest supercomputers at the National Institute for Computational Sciences, Karniadakis and colleagues found that malaria-infected red blood cells stiffened as much as 50 times more than healthy red blood cells. The result: Infected red blood cells, having lost their elasticity, could no longer pass through capillaries, effectively blocking them.
“Basically what happens is the brain could be deprived of nutrients and oxygen,” said Karniadakis, a member of the Center for Fluid Dynamics, Turbulence and Computation at Brown. “This happens because of the deformation of these red blood cells.
“This shows that as stiffening increases (in red blood cells), the viscosity of the blood increases, and the heart has to pump twice as much sometimes to get the same blood flow,” Karniadakis added.
The researchers also found that infected red blood cells had a tendency to stick, flip, and flop along the walls of blood vessels — unlike healthy blood cells that flow in the middle of the channel. For reasons not entirely known, the infected red blood cells develop little knobby protrusions on their cellular skin that tend to stick to the surface of the blood wall, known as the endothelium. Scientists call the sticking cytoadhesion.
“So, what happens is the infected red blood cell is not only stiffer, it’s slowed down by this interaction (cytoadhesion),” Karniadakis said. “This drastically changes the flow of blood in the brain, especially in the arterials and in the capillaries.”
Dimitry Fedosov, first author on the paper, worked on the research as a graduate student at Brown. He is now a postdoctoral researcher at the Institute of Solid State Research in Germany. Bruce Caswell, professor emeritus in the School of Engineering at Brown, contributed to the research. Subra Suresh, former dean of the engineering school at MIT and now director of the National Science Foundation, also contributed to the research.
The National Institutes of Health and the NSF funded the research.