2000
Six Brown University scientists plan to explore the function of the human brain using tiny electronics — nanotechnology — with a $4.25-million grant from the U.S. Department of Defense.
2001
Cyberkinetics Neurotechnology Systems Inc. launches under a licensing agreement with the Brown University Research Foundation, based on research and technology developed in part in the lab of neuroscientist John Donoghue, director of the Brown University Brain Science Program.
2002
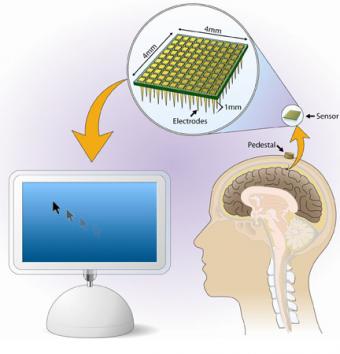
Researchers at Brown University show that signals from the brain, which normally control hand movement, can be decoded and used as the sole input to control a computer cursor. Their report appears in the March 14 issue of Nature.
www.brown.edu/news/2001-02/01-098.html
2004
The BrainGate™ Neural Interface System receives an investigational device exemption from the U.S. Food and Drug Administration, which allows Cyberkinetics to begin testing the device in patients.
2004
Brown and the Providence VA Medical Center receive a five-year, $4.75-million grant to form the Brown-VA Center for Restorative and Regenerative Medicine. The new venture is focused on rebuilding, restoring, and regenerating function for veterans after limb loss.
2005
Brown and Cyberkinetics sign a research and licensing agreement, allowing access for eligible neuroscience researchers at Brown to human clinical data gathered during testing of the BrainGate™ Neural Interface System.
www.brown.edu/news/2005-06/05-012.html
2006
A team led by Brown and Massachusetts General Hospital researchers publish early pilot clinical trial results that show the BrainGate brain sensor allowed a person with tetraplegia (paralysis of both arms and both legs) to open a prosthetic hand, control a robotic limb and move a computer cursor — using thoughts alone. The work was featured on the cover of Nature.
http://www.brown.edu/news/2006-07/06-002.html
2007
Brown and research partners at Cyberkinetics and the Cleveland Functional Electrical Stimulation Center agree to develop new brain implants that record or stimulate neural activity which may be used in patients with paralysis, epilepsy and other central nervous system injuries and disorders.
news.brown.edu/pressreleases/2007/08/next-generation-neurotechnology
2009
The U.S. Food and Drug Administration provides an investigational device exemption to begin a new BrainGate trial. Dubbed BrainGate2, the trial will be run at the Massachusetts General Hospital, with Brown, MGH and the VA Medical Center all playing vital roles in the research.